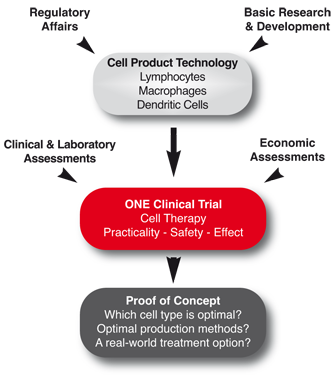
Project objectives
Work Block 1: Cell product development
The aim of Work Block 1 is to obtain licensing for different T regulatory cell, tolerogenic DC and suppressive macrophage cell products. Furthermore, an innovative cell tracking technology will be developed to assess where and for how long cells traffic when administered to humans.
Work Block 2: Trial development, performance, management and evaluation
Work Block 2 is aimed to comprehensively design and conduct a cell therapy based clinical trial in renal transplantation, taking into consideration ethics, concurrent immunosuppressive drug use, state-of-the-art immune monitoring, innovative "all-in-one" data capturing systems, and pharmacovigilance. We expect to deliver a clear and efficient comparative evaluation of haematopoietic cell therapy safety and promise in renal transplantation.
Work Block 3: Understanding and improving immunomodulatory cell types
The aim of Work Block 3 is to learn more about the specific comparative characteristics of suppressive cell types and to use this knowledge to improve later trial designs and foster novel ideas for new or improved suppressive/tolerogenic cell populations. We also aim to support the development of cell populations with a high potential for being used to promote tolerance, but require more than three years to develop into a cell product for cell therapy.